Describe the Nuclear Model of the Atom
AMU stands for atomic mass units used to describe the masses of atoms and subatomic particles. 1 AMU is equal to 112th the mass of a carbon-12 atom at the ground state.

Rutherford Nuclear Model Of The Atom Youtube
Nuclear chemistry is the study of reactions that involve changes in nuclear structure.

. The chapter on atoms molecules and ions introduced the basic idea of nuclear structure that the nucleus of an atom is composed of protons and with the exception of latex_11textHlatex neutrons.

Atom Rutherford S Nuclear Model Britannica
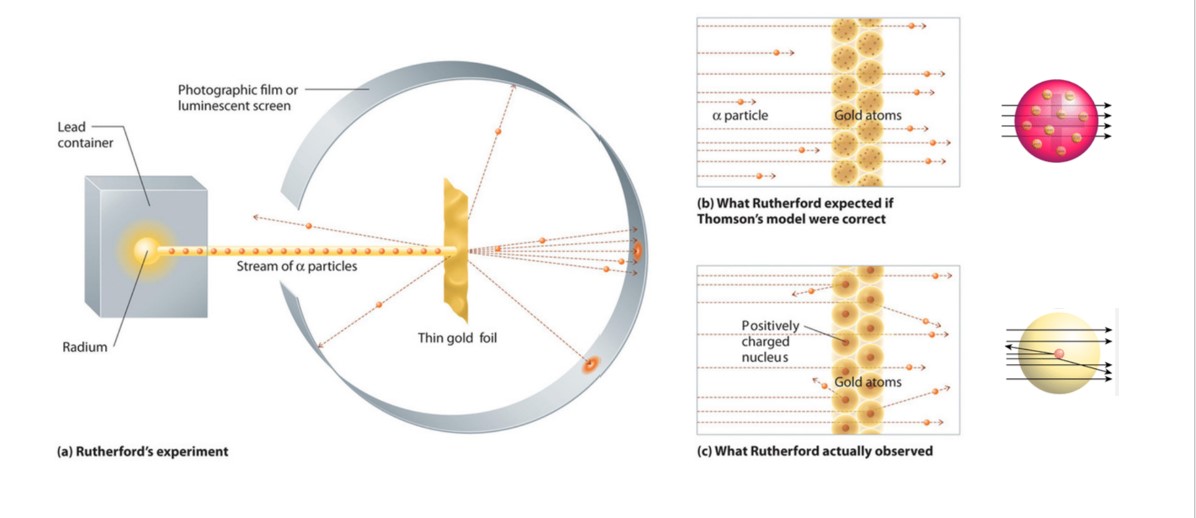
3 4 Rutherford S Experiment The Nuclear Model Of The Atom Chemistry Libretexts


0 Response to "Describe the Nuclear Model of the Atom"
Post a Comment